Constant Surface Tension Boundary Condition
Surface tension and interfacial tension arise because of the differences in attractive intermolecular forces at gas-liquid and liquid-liquid interfaces, respectively. For clarity, the following discussion emphasizes surface tension at gas-liquid interfaces; however, the results are equally applicable to interfacial tension at liquid-liquid interfaces
as a gas-liquid interface is approached from the liquid side, the attractive forces are not felt equally because there are many fewer liquid-phase molecules near the interface. Thus there tends to be an unbalanced attraction toward the interior of the liquid on the molecules near the gas-liquid boundary.
This unbalanced attraction leads to surface tension and a pressure increment across a curved gas-liquid interface that must be properly accounted for when conserving fluid momentum
Attractive forces between molecules at surface and in interior of a liquid for a plane liquid-gas interface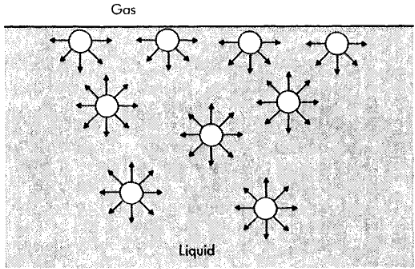 Static equilibrium of a liquid drop in a gas at line of contact with a horizontal solid surface
Static equilibrium of a liquid drop in a gas at line of contact with a horizontal solid surface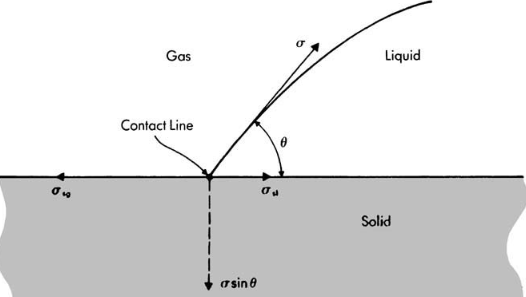 Translation from left to right of a liquid wedge interface
Translation from left to right of a liquid wedge interface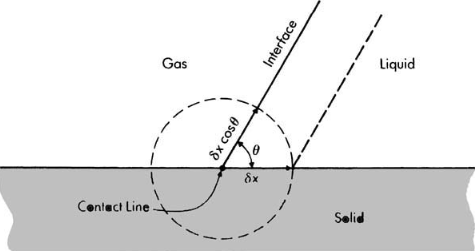 Liquid rise in an open capillary
Liquid rise in an open capillary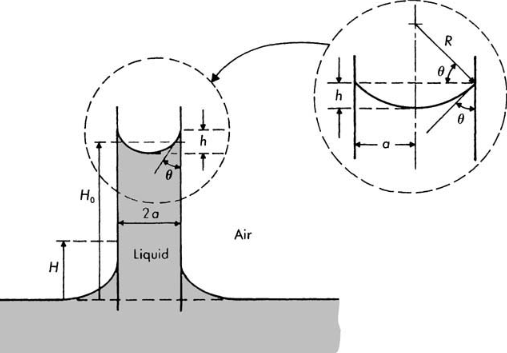
1 Ronald F. Probstein. (1994). Surface tension (pp. 305-361). Physicochemical Hydrodynamics. John Wiley & Sons.
https://doi.org/10.1002/0471725137.ch10